
Scaleup, Equipment Design and Process Optimization for Pharmaceutical and Biopharmaceutical Industry
Typical pharmaceutical and bio-pharmaceutical manufacturing has two broader steps, drug substance (Active Pharmaceutical Ingredient, API) production followed by drug product manufacturing. General steps in API production involve reactions, extractions, distillations, crystallizations, filtration, washing and drying. Drug product manufacturing involves blending, dry/wet granulation, drying as applicable, tabletting and coating.
| Equipment used for API Production | Equipment used for Drug Production |
|---|---|
| Tubular reactors for continuous reactions | Powder blender |
| Crystallizers, Stirred tank reactors | High shear wet granulator |
| Fermenter/Bio reactors | Dryer |
| Distillation columns (batch and continuous) | Tablet press |
| Extractors (batch and continuous) | Tablet coater |
| Drying |
- Stirred tanks for batch chemical reactions, tubular reactors for continuous reactions
- Crystallizers
- Fermenter/Bio reactors
- Distillation columns (batch and continuous)
- Extractors (batch and continuous)
- Drying
Equipment used for Drug Production
- Powder blender
- High shear wet granulator
- Dryer
- Tablet press
- Tablet coater
The Issues faced by pharmaceutical industry during production can be summarized as
Due to improper technology transfer or reactor design (scaleup) and operating conditions, undesirable side products (impurity) can be formed leading to unacceptable product quality.
- Undesired particle size distribution
- Loss of physical properties during drying
High impeller speed and/or wrong selection of impeller can lead to unreasonable high shear resulting in particle attrition. Finer particles can lead to compact mix and hence difficult to dry
improper drying would lead to fines or lump formation, side reactions, improper power density, higher utility consumption, particle carryover, higher operating costs, more recycle leading to lower production
The tank banks have very different configuration from the pilot tanks. Thus the standard scaleup rules cant be applied. A lack of understanding of limiting step would lead to incorrect scaleup ( equal P/V, shear, tip speed, kla) and impeller speed.
- Undesired purity profile leading to unacceptable product quality
- Inadequate API physical properties
Undesired particle size distribution (PSD including fines generation)
Loss of physical properties during drying
- Inadequate and inefficient drying
- Unreliable scale-up and technology transfer
Crystallizers
Crystallization plays an important role in the manufacture and purification of small-molecule active pharmaceutical ingredients (APIs). It is estimated that between 70 and 80% of all small-molecule, APIs have at least one crystallization step in their manufacturing processes. To facilitate other downstream processes, such as filtration, drying, dissolution testing, and formulation, it is often desirable to be able to consistently produce crystals with specific properties, such as crystal size distribution, crystal shape (habit), and polymorphic form.
In the case of crystallization processes, reliable scale-up is heavily dependent upon the reproducibility of both local and global mixing characteristics across laboratory to pilot to commercial scale.
Fermenter/Bioreactors
Bioreactors/fermenters are typically gas-liquid stirred tank reactors designed to encourage microorganisms to thrive, allowing sufficient fermentation to create a usable end product.
A number of criteria must be satisfied when a bioreactor is built. In order for the device to be effective, following conditions need to be tightly controlled.
- Adequate aeration
- Low shear
- Mixing uniformity
- Uniform gas dispersion
- Better pH Control
- Efficient CO2 Stripping
Impeller configuration, Speed of rotation, sparger design, sparger location with respect to the impeller plays a critical role in the cell viability, cell count, cell growth, pH control, p_CO2, p_O2 in the bioreactor.
As bioreactors/Fermenters can have working volumes as small as a few milliliter to up to several thousand liter, it is often differentiated between lab-scale, pilot scale and manufacturing scale bioreactor systems.
A balance of mixing, gas dispersion, bubble size, shear levels, and kla needs to maintained at all the scales. The basic scaling principles of geometric similarity can be a good guideline for scaleup. However, the reactors at different scales following scaleup principles is not always possible.
A detailed process model of the bioreactor coupled with CFD simulations can ensure optimal operating conditions at different scales to ensure same product quality and smooth operations.
Scale-up and Scale-down
Scaleup and Scale down is one of the critical aspect for Pharma Industry.
A well-known source of bottlenecks, delays, and technical setbacks is process scale-up, where production moves from the laboratory to full-scale GMP manufacturing.
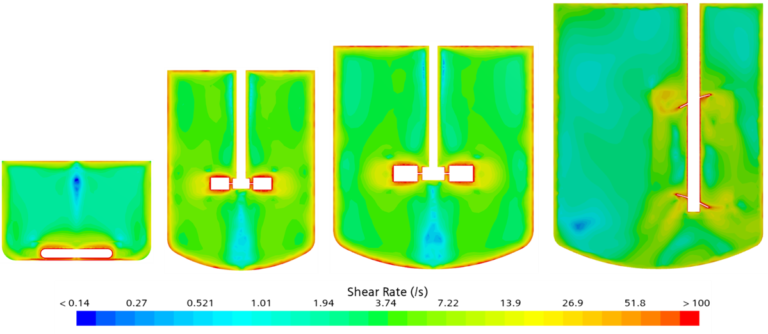
The laboratory experiments are generally carried out in 50ml liquid volumes ( 250m ml flasks) stirred with magnetic beads. as the scale of operation/experimentation is increased from few milliliters to few liters and more, availability of reactors with perfect scaleup rules becomes a challenge. For example, the laboratory and scaleup teams could have tanks with Rushton impellers and pitched blade down pumping impellers with liquid height to tank diameter ratio of unity. Whereas the larger reactors would be tall with multiple impeller systems. The tall vessels typically have combination of paddle type and 6-Bladed Rushton turbines. The impeller shaft could be offcentered. This leads to different hydrodynamics, mixing patterns, shear levels, gas dispersion across the scales.
Due to a range of unique rheological challenges, scale-up has been particularly difficult for manufacturers of solid-dose (Crystallization) pharmaceuticals. It is equally important for the fermenter scaleup as pH Control on various scales is important. Achieving homogeneity of mix at all the scales is another critical issue. Segregation could lead to out of spec product.
Computational Fluid Dynamics studies gives a detailed insight of hydrodynamics at different scales. This information is then extended to predict O2 and CO2 levels and their distribution in the tank. Now the task is only to optimize the operating conditions to ensure all the critical parameters are similar at all the scales!


Batch to Continuous
There has been growing interest to convert the traditional batch processes to continuous processes for both the quality and efficiency reasons.
Typical batch feeding, preheating, heating, reaction time, cooling steps that are carried out in a batch stirred tank now can be performed in tubular reactors. The tubular reactors now need to be long enough to ensure preheating, effective mixing of the ingredients during the flow, provide required residence time for reaction, maintaining controlled temperature conditions during reactions and rapid cooling after reaction.


From the design perspective, the surface areas and flow velocities, length of tubular reactors, mixing zones, heat exchange have to be designed while maintaining the production targets.
The traditional experimental methodology to accomplish the switch from Batch to continuous can be extremely time consuming and less economical while incurring wastage of materials and loss of resources.
FDA has been encouraging to use “Quality by Design” principles to define the process design space, guide risk assessment and control strategy.
In the QbD paradigm, mathematical models can potentially be used at every stage of API and drug product development and manufacturing
Modeling can help establish a predictive framework using experimental data and scientific principles to create mathematical representation of the system.
Process Modeling
Predictive models aid process design by
- Evaluating the impact that unit operations/processes, equipment, and material inputs have on product attributes
- Providing a framework for risk assessment, process control, and optimization, where accurate predictions of the system are required


Optimization and Risk Assessment
At the design stage, flowsheet model is a very powerful tool to evaluate equipment configurations and manufacturing schemes at a much lower cost than the equivalent experimental investigation. Using flowsheet models, the major routes for drug substance and drug product manufacturing can be evaluated, and challenges with process scale-up can be anticipated and resolved. It can be used as a tool for process design, optimization, risk assessment, control strategy analysis, and monitoring of a continuous pharmaceutical process.
Controls system design and evaluation
The input parameters, material properties, operating conditions cannot be expected to be steady. Any variation in the input parameters results in variation of the product properties. FDA suggests Pharma companies to define the product quality matrix and acceptable variation. Thus, it is important to critically examine the impact of variation in each of the input variable on final output. Process Models and the flow sheet models allow to study the impact of input variability on the output. The cascading impact of input variation on the entire flow sheet can only be analysed through flow sheet modeling. This helps in designing an appropriate process control system.
Computational Fluid Dynamics
An important strength of Computational Fluid Dynamics (CFD) Model is to evaluate the performance of various process equipment configurations that enable us to choose the right design to achieve desired product quality and throughput. Now that variations of micro reactors are available, a lot of Pharma companies have started to explore CFD modeling approach to select a right design to meet their requirements. There has been a realization that the experimental route to process equipment selection is very time consuming and expensive. Once the design is shortlisted using CFD, the design is verified by conducting appropriate experimentation.
How can we Help?
FluiDimensions has experience of reactor modeling, flow sheet modeling and CFD. We use standard platforms for flow sheet modeling. We also develop and design programs for specific applications using programming languages like FORTRAN, C, C++, Python. We have the expertise to create GUI to enable seamless use of intricate models for various applications.
We have strong capability in modeling of complex physics involving multiphase flows, mass transfer, heat transfer, chemical reactions and dynamic particulate systems.
We offer our expertise and services to
- Predict the equipment performance at scale-up
- Suggest operating conditions to achieve performance as obtained in laboratory scale
- Design of reliable scale-up
- Evaluate Equipment performance before procurement
- Perform parametric sensitivity of operating conditions and predict reactor/process performance
- Analyze the complete process flow sheet. Steady and dynamic analysis and optimize the process conditions
